Beyond Insulin: Potential Cures for Type 1 Diabetes
Continuing from my previous article, here is another one in which I will explore several potential/ experimental treatments for type 1 diabetes (T1D). Insulin replacement has always been the only treatment for patients with T1D, and there is still no one-time cure for diabetes. That is why I wanted to find out about novel ideas for treating this disease.
Reprogramming Alpha Cells
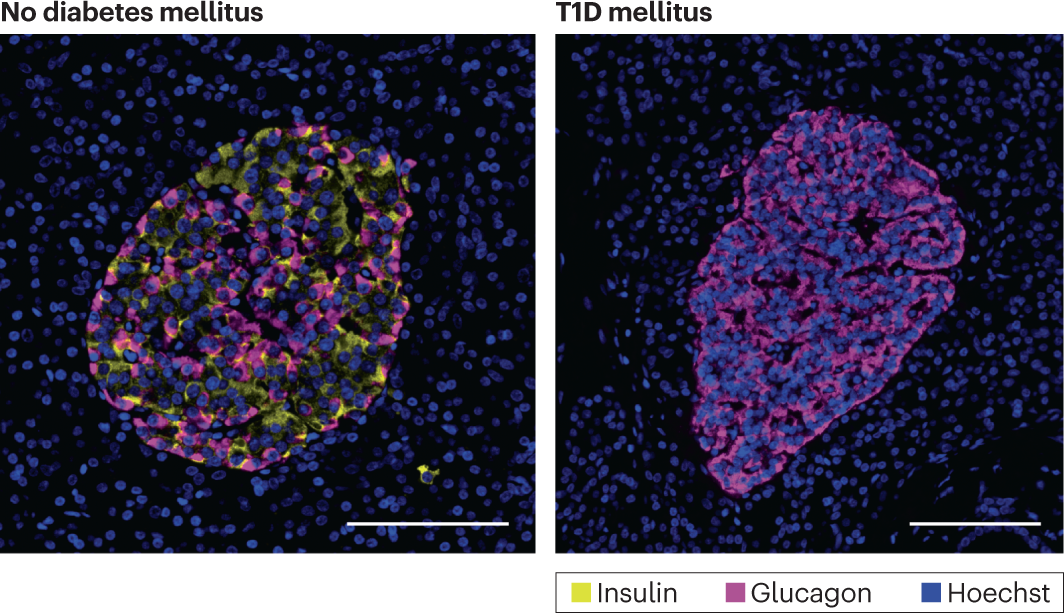
Another type of cell in the pancreas is the alpha cell. Unlike the beta cells that produce insulin, alpha cells produce glucagon, a hormone responsible for raising blood glucose levels by breaking down glycogen (GCG) stored in the liver into glucose. Since alpha cells can still produce GCG when a person has T1D, their blood glucose level will be unregulated, leading to hyperglycaemia. In the islets of Langerhans (a cluster of cells in the pancreas) of a non-diabetic person, beta cells are primarily found in the centre, with alpha cells and other hormone-excreting cells surrounding them. Using NOD mouse models (non-obese diabetic mice used to simulate diabetic humans), it is observed that the beta cells of the islet centre are replaced by an increased number of mostly alpha cells and some other cells, such as delta cells. (Hoechst is just a dye for staining DNA in cells)
Usually, beta cells contain the transcription factors Pdx1 and MafA, which allow genes responsible for the differentiation of beta cells and insulin production to be activated. Since alpha cells are found to be more present in the islets of Langerhans, placing the genes in the alpha cells through gene therapy was a widely discussed theoretical method. The genes would be inserted using an adeno-associated virus (AAV) vector by entering the alpha cells through endocytosis; the virus would then go through a nuclear pore, then the DNA would exist as an episome, which is a loop of DNA closed off from the human genome (like a plasmid), capable of expressing its genes independently. Transcription and translation of the genes would produce the insulin required for diabetics. AAVs have different serotypes (strains), with each serotype having its own tropisms and, therefore, could be utilised for infecting specific cell types; for example, AAV2 has a high affinity to liver and skeletal muscle cells, AAV9 can cross the blood-brain barrier for gene therapy to neuronal cells used in essential breakthrough treatments like Zolgensma for Spinal muscular atrophy and AAV8 having a high affinity to pancreatic cells, therefore, is used for purposes like this.
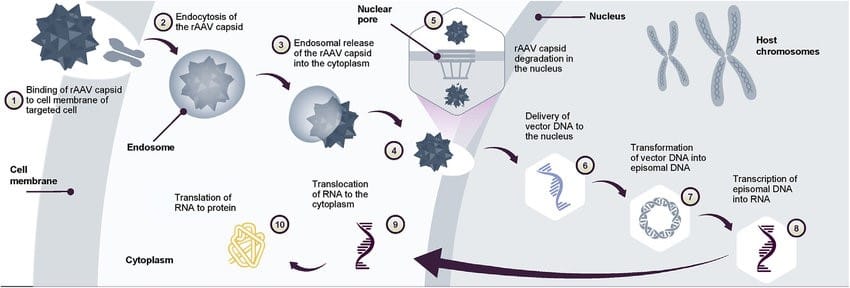
A study showed that testing this method on the NOD mouse models yielded a 95% success rate in delivering the genes as identified by GFP (green fluorescent protein). However, when the genes were delivered without a PM (polyomavirus middle), the glucose level did not normalise. A polyomavirus middle is a promoter allowing transcription factors to bind to it for controlled gene expression; it is delivered to the nucleus along with the gene. The whole delivery package is known as AAV8-GCG-PM; the AAV8 being the adeno-associated virus vector of serotype 8; the GCG allowing the treatment to be specific to the alpha cells; and the PM driving the expression of the PDX1 and MafA regulated genes. It was found that the blood glucose level normalised after 5 weeks of the viral infection, and the mice could control their blood glucose level for at least 7 months after the treatment. This raised the possibility that the immune system did not recognise the transgenic alpha cells as beta cells, allowing them to evade autoimmune attacks. This treatment would be aimed at patients with the early development of T1D when the beta cells are destroyed, and the alpha cells start to proliferate and occupy more of the islet; some of the alpha cells would be infected, causing the infected alpha cells to proliferate along with normal ones, potentially reversing the effects of T1D. With the success of the trials in rodents, the next step is to aim to extend and test how long the efficacy could be maintained and test the treatments on humans.
Tregs
As mentioned in the previous essay, T1D is an autoimmune disease; most destruction of beta cells is caused by T-cells. Utilising regulatory T-cells (Tregs) is a potential way to suppress their activities. Treg cells regulate T-cells (as shown in their name): They have the ability to release anti-inflammatory cytokines such as IL-10, the ability to release granzymes and perforins (their functions are explained in my previous essay) and have receptors which are complementary to cytokines responsible for activating cytotoxic T killer cells, acting basically like a sponge, inhibiting them from being activated. Tregs are utilised hugely by cancer cells to evade immune detection or even cause T-cell apoptosis. Currently, there are many potential methods involving Tregs to cure T1D, including exogenous Treg therapy, CAR Treg therapy, and antigen-specific treg therapy.
Exogenous Treg therapy
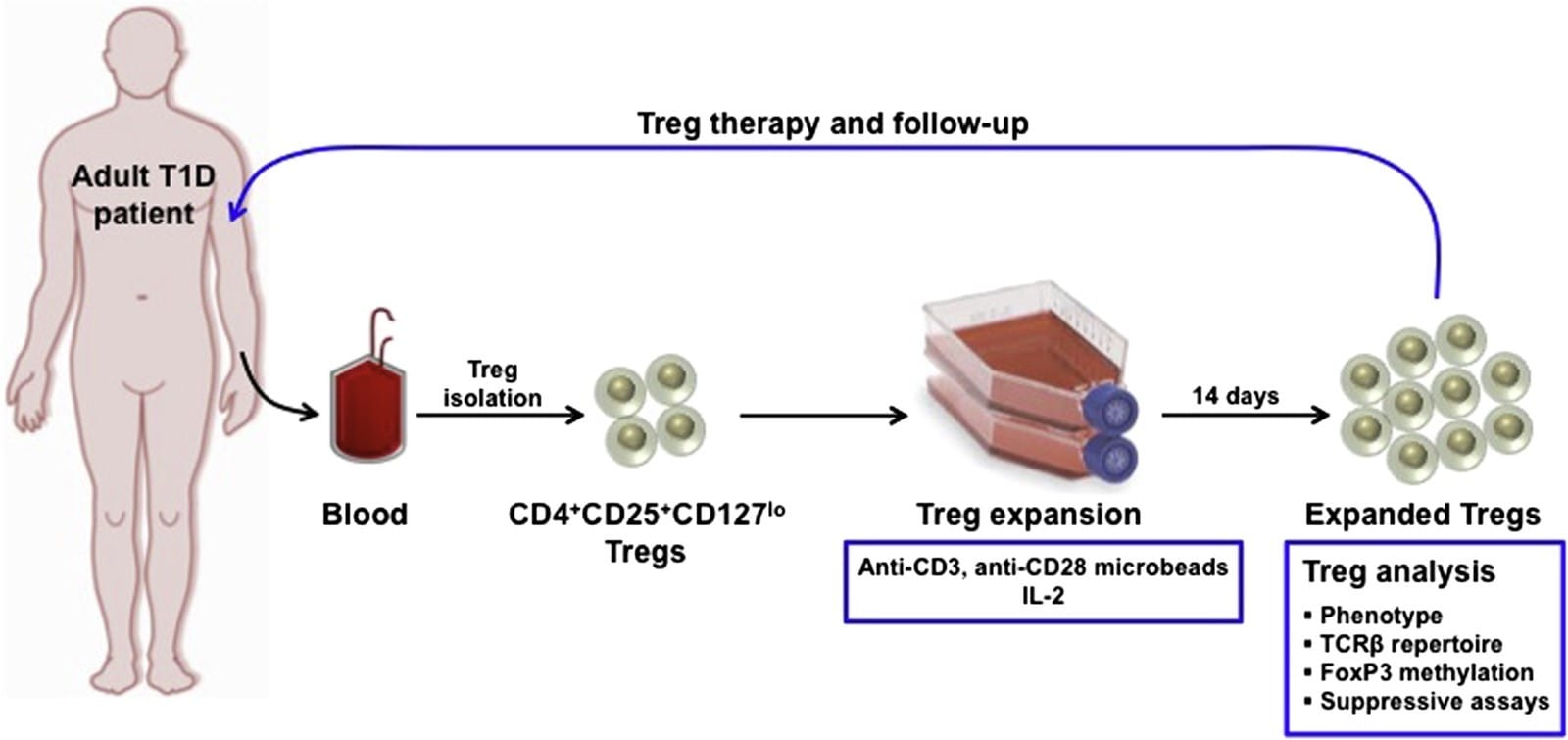
Exogenous Treg therapy is a potential way to increase the number of Tregs. Treg cells are extracted from the patient’s blood. They are cloned and modified using microbeads, which are small beads coated with specific surface receptors (like CD4) and antibodies to stimulate the growth and proliferation of the specific Treg cells; cytokines such as IL-2 are also added to promote cloning. Released by a helper T cell, IL-2 is normally an inflammatory cytokine triggering T cells to undergo clonal expansion and differentiate into even more helper/ killer/ Treg/ memory cells. Then, the separation process occurs. Using magnets, the Tregs are separated from the rest of the unwanted cells as they grow on the surface provided by the microbeads. The expanded (cloned) Tregs would then be put back into the patient through an intravenous injection; they could then secrete anti-inflammatory cytokines and down-regulate T-cell activity, restoring the immune tolerance eventually so that the T-cells will stop attacking the beta cells.
The potential downside of this method is the contamination of the expanded Tregs with pro-inflammatory cells like T-helper cells, which would then be injected back into the patient, countering the effects of the Tregs. Testing this method on NOD mouse models showed that it is safe; however, its efficacy is still being evaluated. There is also currently ongoing research on strategies to improve the culturing of Tregs.
CAR-Treg Therapy
You might have heard of CAR-T therapy in immunotherapy, as it is one of the most groundbreaking innovative techniques in combatting haematological cancers such as leukaemia. Although expensive, this treatment has a high success rate and limited side effects, making it very promising. (If you want to find out more about CAR-T therapy, you can ask Ryan from Lockites to explain; he has done a presentation about it for Med-Soc)

CAR-Tregs therapy works similarly to CAR-T; usually used to restore immune tolerance and combat allotransplantation rejection (human-to-human transplantation), CAR-Tregs have been shown to work on autoimmune diseases as well. The premise is to amplify the immune suppression effects and to enhance the specificity through genetically modifying Tregs obtained from the patient. The process of creating CAR-Tregs involves getting blood from the patient first, then separating the Tregs and then allowing them to proliferate in vitro and putting them back into the patient, often through a one-time IV injection.
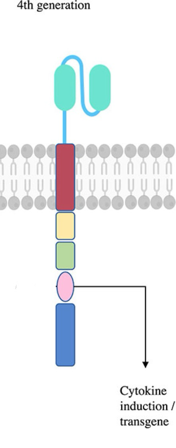

Like CAR-T cells, CAR-Tregs contain an intracellular domain and an extracellular domain; the intracellular domain, using intracellular cell signalling mechanisms, is responsible for improving the effectiveness of the cell. It initiates a cascade that promotes the upregulation of cytokines such as IL-10 and TGF-β (another cytokine that causes a positive feedback loop to transform T-helper cells into Tregs) and boosts the Treg's proliferation and resistance to apoptosis. The extracellular domain allows the CAR-Tregs to bypass the activation of major histocompatibility complexes (MHCs). Normally, MHCs are required for any T-cells to be activated; for example, a cytotoxic killer T-cell has to bind to MHC class Is, which are present on every single cell and determine if the cell is functioning normally or not. CAR-Tregs could be activated without needing to bind to one; therefore, as soon as these new cells are reintroduced to the patient, they could recognise a wider variety of antigen types and start doing their jobs much quicker. The extracellular domain could also be altered to target specific antigens since different autoimmune diseases might have different antigens responsible for the symptoms (for T1D, it’s cytotoxic T-killer cells).
Antigen-specific Treg therapy
This treatment has a relatively basic concept. Tregs, like T-cells, are also activated by antigen-presenting cells. When they encounter the MHC, they induce tolerance to the specific antigen they were presented to. They will go to the inflamed site, release cytokines, and inhibit T-cell activities.
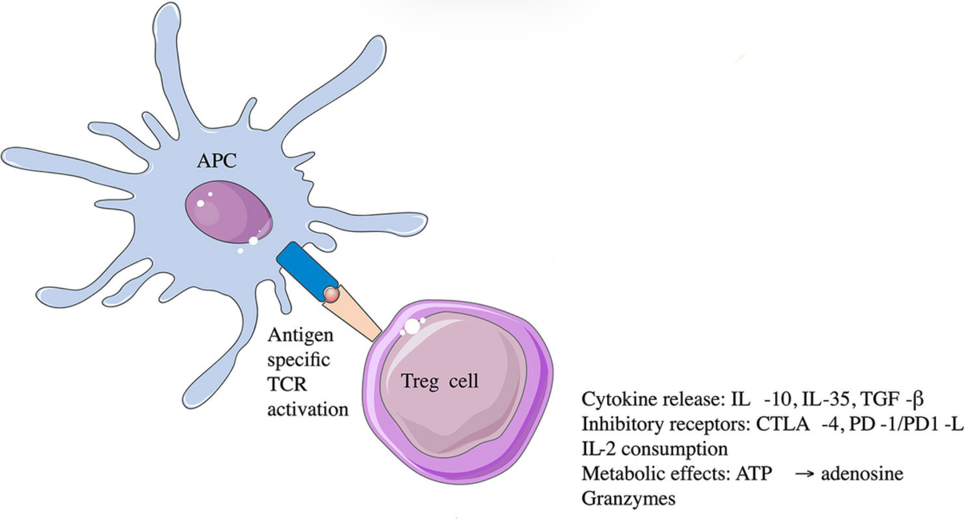
By presenting antigens that trigger a response to Tregs, the body builds up a tolerance against the antigen over time. If we artificially present antigens that trigger autoimmune T-cells to Tregs, the immune tolerance to beta cells could be increased. The antigens that activate the immune response could be insulin or other specific proteins found uniquely on the cell surface membrane of beta cells.
In vivo studies show a delay in the onset of symptoms in children with T1D. This treatment failed to achieve its primary objective, which is curing T1D, and scientists are still trying to figure out the mechanisms behind the failure. Theories include the impairment of the induction of Tregs with the specific antigen (insulin) and the instability of a transcription factor, FoxP3, in environments of inflammation. (FoxP3 is responsible for the development, maintenance and functioning of Tregs)
There are many other potential treatments for T1D, but here are a few that I found quite interesting. Tregs have immense potential to treat various autoimmune diseases, and hopefully, time will allow science to cure the incurable.
