How Small Changes Can Make a Big Difference in Chemical Structures
A chemical name or structure must be specific to a chemical compound. But what difference does it make if you slightly change that chemical name or structure? It turns out that due to the intricacy of chemical nomenclature, geometric isomerism, optical isomerism, and various carbon chain lengths, two very similar compounds can have drastically different properties.
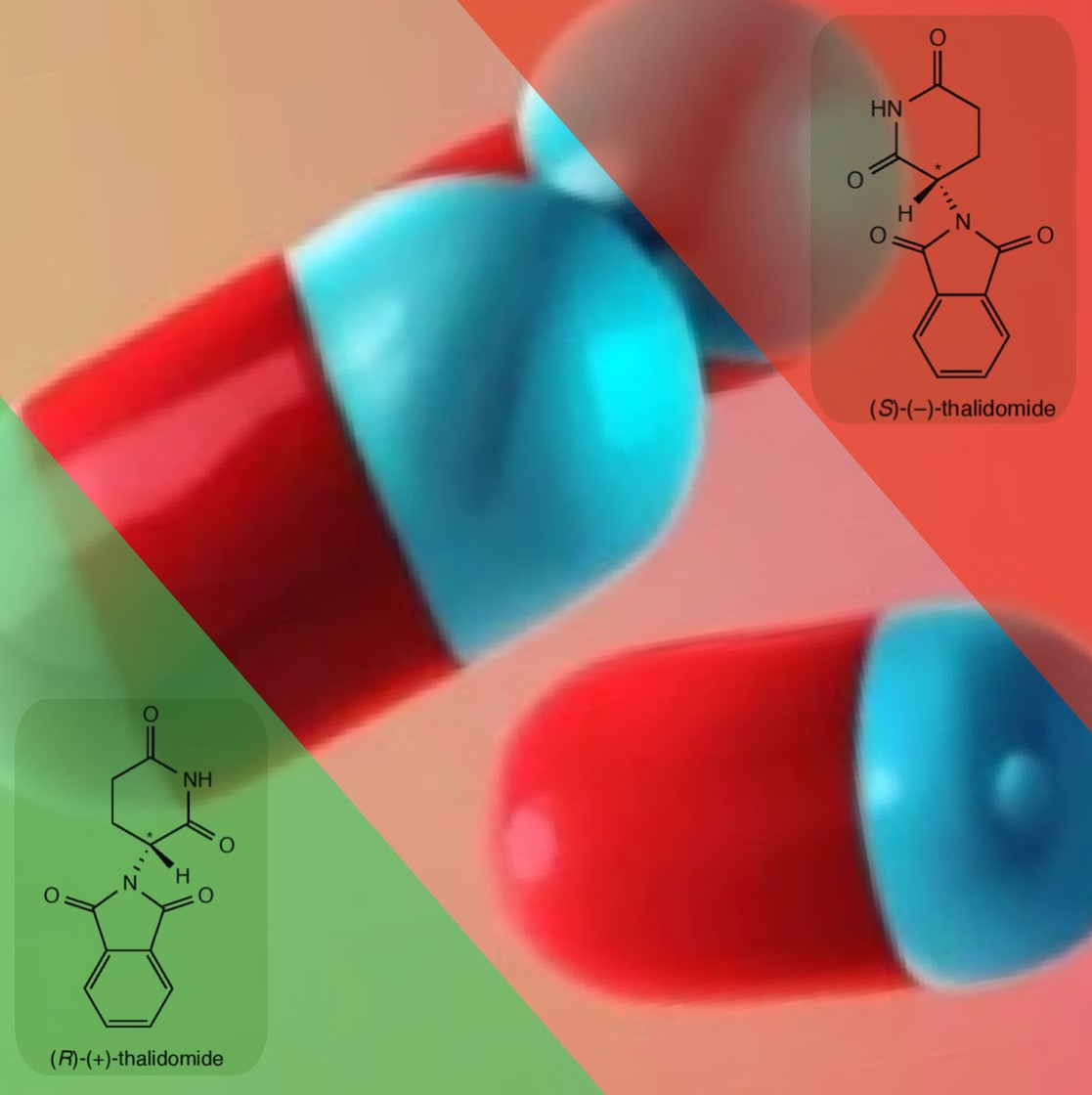
An example of how a minor error in a chemical structure can have tragic consequences is the Thalidomide Tragedy. Thalidomide is a pharmaceutical drug that was introduced by the German drug company Grünenthal in 1957 to treat morning sickness in pregnant women. The IUPAC (International Union of Pure and Applied Chemistry) name for the prescribed drug is (RS)-2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione. By focusing on the first two letters of this complicated chemical name - the (RS) part - it indicates that an equal mixture of enantiomers was prescribed, which is called a ‘racemic mixture’ or ‘racemate’. The R/S naming system indicates how the four different groups, bonded to the chiral carbon, are arranged. The figure below shows that the enantiomers of thalidomide are chiral molecules that are non-superimposable mirror images of one another.
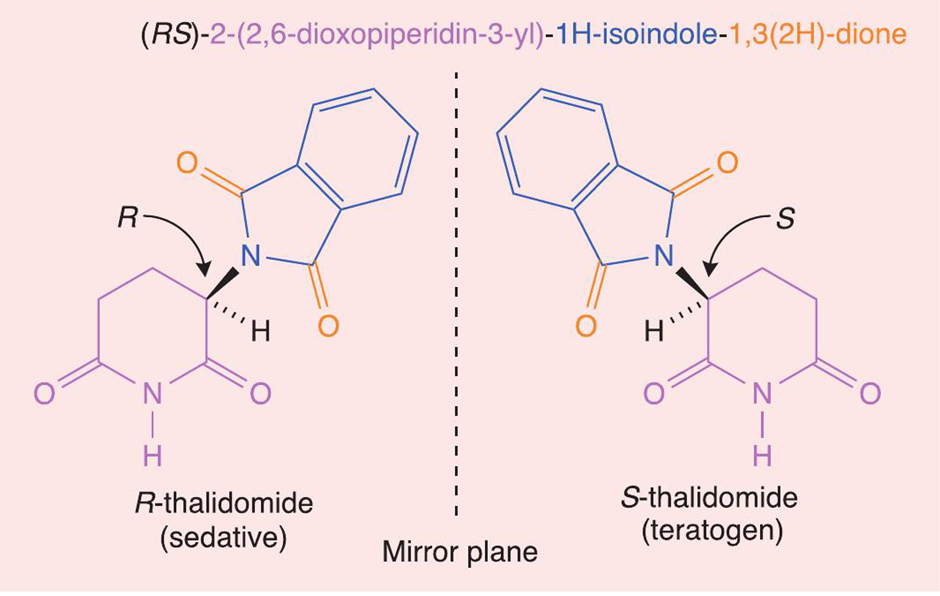
In the late 1950s and early 1960s, it was not realised that although the R-enantiomer had the desired sedative effect to suppress morning sickness, the S-enantiomer was a teratogen, which is an agent that is able to interfere with the development of an embryo fetus, causing severe birth defects. The S-enantiomer inhibits new blood vessel growth in the foetus, which is critical for the development of limbs and other organs. As a result, around 10,000 infants worldwide were born with phocomelia, or limb malformation, before thalidomide was taken off the market in the early 1960s. Only 50% of the infants survived, and some of those who did had other defects in addition to limb deficiencies.
You may think that a feasible solution to this problem would have been to isolate the R-enantiomers of thalidomide and sell this pure form as the drug. However, the reason why this prescription would not be safe is that the liver contains an enzyme that is able to interconvert the optical isomers and so the R-enantiomer would be transformed into the S-enantiomer. Therefore, even if an enantiomerically pure drug were administered, the body would convert it into a racemic mixture, which would still result in the harmful side effects. This is why pregnant women should never be exposed to either the R-enantiomer or S-enantiomer of the drug.
Furthermore, it is evident that a change in the way the same groups are bonded to a chiral carbon affects biological activity, which is due to the R- and S-enantiomers having different shapes. One hypothesis that can help to explain this difference in biological activity is the lock-and-key model. Within the body, drugs like thalidomide interact with enzymes. Enzymes are proteins that are biological catalysts, which increase the rate of biochemical reactions. They are relatively large molecules with complicated three-dimensional shapes, which allow specific molecules, called substrates, to fit into them and bind to them. The area to which they bind is called the active site. When certain drugs bind to the active site, it stops the enzyme from functioning normally as the drug blocks any substrate binding to the active site. In the lock-and-key model, for a drug to bind to an enzyme, the shape of the drug must have a complementary shape to the active site. Only the appropriately shaped drug will fit, just like how only one correctly shaped key will fit into its specific lock. In 2010, a team of researchers at the Tokyo Institute of Technology showed that when the enzyme cereblon stops working normally in female zebra fish, developmental malformations occur in the offspring. These effects were similar to the human birth defects caused by administering thalidomide. This research indicated that the birth defects are caused by S-thalidomide blocking the normal action of cereblon. Subsequently, in 2018, the S-enantiomer of thalidomide was shown to bind to cereblon more strongly than the R-enantiomer. Since the R- and S-enantiomers of thalidomide have different shapes, they can interact differently with the same enzyme, which is why they have different biological effects.
Although this thalidomide scandal had tragic consequences, one positive outcome of it was that it caused many countries to tighten drug approval regulations, making the medicines we take today much safer. Thalidomide is also still a valuable drug today for certain conditions, such as treating multiple myeloma (a form of blood cancer) and leprosy.
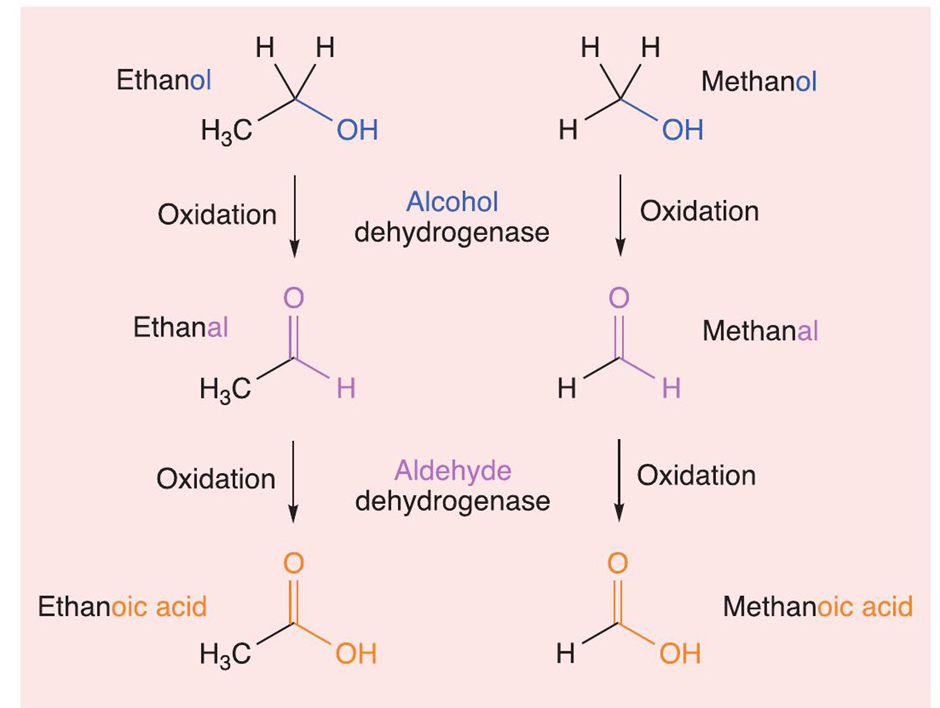
The thalidomide tragedy is a reminder of how important even minor changes in the chemical structure of a drug can be. However, it is not just pharmaceuticals - a small error in the structure or name of a number of everyday organic chemicals can have adverse consequences. For example, compare methanol and ethanol: only the letter ‘m’ distinguishes their names and there is only a CH₂ (methylene) group that differentiates their structures. Methanol has the structural formula CH₃OH, whereas for ethanol it is CH₃CH₂OH. However, while ethanol is the intoxicating ingredient in wine and beer, methanol is a chemical that becomes highly toxic when processed, or metabolised, by the human body. Consuming only 4-10 ml of methanol can cause blindness, and a lethal dose can be as small as 30 ml. For ethanol, the lethal dose is higher.
Many people turned blind or died during Prohibition in the USA, as people made their own ‘moonshine’ which contained high levels of methanol. But what makes methanol more toxic than ethanol? The metabolism of ethanol starts with an enzyme called alcohol dehydrogenase in the liver. This enzyme oxidises ethanol into ethanal, as shown in the figure below. Ethanal is one of the chemicals that causes the symptoms of a hangover. This aldehyde is further metabolised into ethanoic acid (or acetic acid, i.e. vinegar), and then into harmless carbon dioxide and water. In comparison, although alcohol dehydrogenase metabolises methanol into methanal (or formaldehyde), this is a slower process than the oxidation of ethanol. This slower oxidation process is why the symptoms of methanol poisoning can take hours or days to become apparent. When methanal is metabolised, it is oxidised to form methanoic acid (or formic acid, which is found in ant stings). This carboxylic acid is also slow to metabolise. High levels within the body have been linked to blindness, as it accumulates in the optic nerve, and can eventually cause organ damage and death.
Changing the length of the carbon chain in a molecule is also an important consideration in the chemicals we use in our daily lives. For example, the plasticiser 1,5-pentanediol, HOCH₂CH₂CH₂CH₂CH₂OH, has 5 carbons, but this can be changed to a similar compound 1,4-butanediol, HOCH₂CH₂CH₂CH₂OH, which has just four carbons. Plasticisers are used to soften plastic products and make them more flexible. 1,5-pentanediol was used as a plasticiser in Aqua Dots, a children’s arts and crafts toy. In 2007, 4.2 million units of the toy were recalled because five children were hospitalised after swallowing the beads. The manufacturer had used 1,4-butanediol in place of non-toxic 1,5-pentanediol, perhaps because it was a much cheaper chemical. However, 1,4-butanediol is dangerous as when it is ingested into the body, it is converted into 4-hydroxybutanoic acid (or gamma-hydroxybutyric acid, GHB): HOCH₂CH₂CH₂CO₂H. Similar to the oxidation of methanol and ethanol as before, an alcohol group is oxidised to a carboxylic acid within our bodies. However, GHB has a very similar structure to 4-aminobutanoic acid (or GABA): H₂NCH₂CH₂CH₂CO₂H. GABA is the main inhibitory neurotransmitter in our body. It transmits an impulse from one nerve cell to another and it has a relaxing effect. Our bodies cannot distinguish between GHB and GABA, which means that GHB also acts as a neurotransmitter. In low doses it causes drowsiness and nausea, whereas at higher doses it can cause unconsciousness and seizures. Conversely, when 1,5-pentanediol enters the body it is converted into 5-hydroxypentanoic acid: HOCH₂CH₂CH₂CH₂CO₂H. Because it has a slightly longer carbon chain, this compound does not act similarly to GABA.
Overall, it is astonishing how meticulous chemical nomenclature and stereoisomerism is, and how important it is for chemists to ensure that they use exactly the correct chemicals in industry, as such a seemingly trivial change can make all the difference in the properties of chemical compounds.